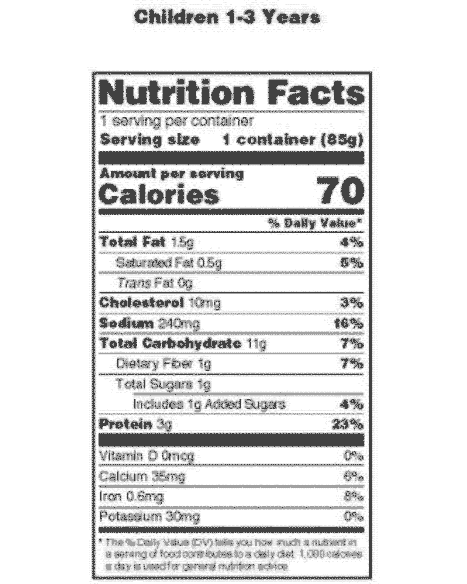
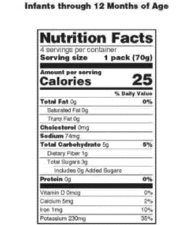
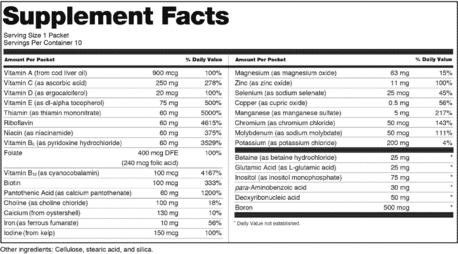
42 information that must be lawfully provided on food labels
› regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims We have published regulations that describe exactly what the disclaimer must say and what you must include in your notification to us and where you must send it in the September 23, 1997 Federal ... Music Creators - ASCAP Two types of music users are exempt, under different standards: a food service or drinking establishment (defined as "a restaurant, inn, bar, tavern, or any other similar place of business in which the public or patrons assemble for the primary purpose of being served food or drink, in which the majority of the gross square feet of space that is nonresidential is used for that …
38. Church Policies and Guidelines - The Church of Jesus Christ … 38.1. Church Participation. Our Father in Heaven loves His children. “All are alike unto God,” and He invites all “to come unto him and partake of his goodness” (2 Nephi 26:33).Church leaders and members are often asked who can attend meetings of The Church of Jesus Christ of Latter-day Saints, who can become Church members, and who can attend a temple.
Information that must be lawfully provided on food labels
› news-events › public-health-focusFDA Regulation of Cannabis and Cannabis-Derived Products ... In addition, under 21 CFR 530.20(b)(2), if scientific information on the human food safety aspect of the use of the approved human drug in food-producing animals is not available, the veterinarian ... › current › title-21eCFR :: 21 CFR Part 812 -- Investigational Device Exemptions When the sponsor receives from the IRB information concerning the public disclosures under § 50.24(a)(7)(ii) and (a)(7)(iii) of this chapter, the sponsor shall promptly submit to the IDE file and to Docket Number 95S-0158 in the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD ... Nutrition Final Exam Flashcards - Quizlet Information that must be lawfully provided on food labels includes all of the following except... The amount recommended for ingestion each day.
Information that must be lawfully provided on food labels. Marijuana Packaging & Labeling Laws by State | Leafly 22 sept. 2015 · Our state-by-state guide to cannabis packaging and labeling laws helps cannabis businesses understand which guidelines they must follow. Small Entity Compliance Guide on Structure/Function Claims We have published regulations that describe exactly what the disclaimer must say and what you must include in your notification to us and where you must send it in the September 23, 1997 Federal ... Techmeme 26 oct. 2022 · The essential tech news of the moment. Technology's news site of record. Not for dummies. Food Labeling Guide - FDA Answer: Food labels must list: a. Name and address of the manufacturer, packer or distributor. Unless the name given is the actual manufacturer, it must be ...
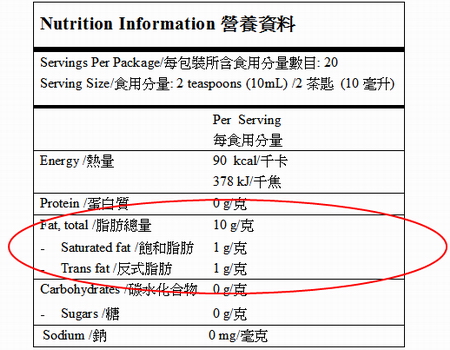
› music-creatorsMusic Creators - ASCAP The name and address of the record company should appear on the record label. The Recording Industry Association of America, a trade organization for record labels, can provide you with more information on the rights of record labels. Recording Industry Association of America (RIAA) 1020 19th St. NW, Suite 200 Washington, D.C. 20036 Tel: (202 ... Food Labelling (Codex Alimentarius) - Fifth Edition schools, hospitals and similar institutions where food is offered for immediate ... The following information shall appear on the label of prepackaged foods ... FOOD LABELING - GovInfo (a) The term information panel as it applies to packaged food means that part ... to be safe and lawful under the applicable food safety provisions of the ... Food Labeling: Revision of the Nutrition and Supplement Facts Labels May 26, 2016 ... Revise the nutrients of public health significance that must be declared on the label. The proposed rule would require the declaration of ...
Statutes & Constitution :View Statutes : Online Sunshine A medical marijuana treatment center that produces edibles must hold a permit to operate as a food establishment pursuant to chapter 500, the Florida Food Safety Act, and must comply with all the requirements for food establishments pursuant to chapter 500 and any rules adopted thereunder. Edibles may not contain more than 200 milligrams of tetrahydrocannabinol, and a … Direct Shipment of Alcohol State Statutes - National Conference of ... 15 oct. 2021 · The order must give reasonable notice of the rights of the person to request a hearing and must state the reason for the entry of the order. Unless otherwise agreed between the parties, a hearing shall be held not later than seven days after the request for the hearing is received by the commissioner after which and within 20 days after the receipt of the … eCFR :: 21 CFR Part 812 -- Investigational Device Exemptions When the sponsor receives from the IRB information concerning the public disclosures under § 50.24(a)(7)(ii) and (a)(7)(iii) of this chapter, the sponsor shall promptly submit to the IDE file and to Docket Number 95S-0158 in the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852, copies of the information … Nutrition labelling - Language selection | Food Safety May 20, 2020 ... The declaration must be presented in a legible tabular format on the packaging. Where space does not permit it, the information may be presented ...
› statutes › indexStatutes & Constitution :View Statutes : Online Sunshine 8. A medical marijuana treatment center that produces edibles must hold a permit to operate as a food establishment pursuant to chapter 500, the Florida Food Safety Act, and must comply with all the requirements for food establishments pursuant to chapter 500 and any rules adopted thereunder. Edibles may not contain more than 200 milligrams of ...
Marriage - Wikipedia Marriage, also called matrimony or wedlock, is a culturally and often legally recognized union between people called spouses.It establishes rights and obligations between them, as well as between them and their children, and between them and their in-laws. It is considered a cultural universal, [citation needed] but the definition of marriage varies between cultures and …
› scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · A reference to information submitted previously must identify the file by name, reference number, volume, and page number where the information can be found. A reference to information submitted to the agency by a person other than the sponsor is required to contain a written statement that authorizes the reference and that is signed by the ...
Quiz 2. Planning A Healthy Diet Flashcards | Quizlet Information that must be lawfully provided on food labels includes all of the following EXCEPT: The amount recommended for ingestion each day.
Current Food Labeling - Nutrition Labeling - NCBI Bookshelf The purpose of this chapter is to describe how food labels are currently ... The regulations specified how nutrition information was to be provided if a ...
Food Labelling for Consumers – EU Law, Regulation and Policy ... FIR Food Information Regulation, Regulation (EU) No 1169/2011 of the European. Parliament and of the Council of 25 October 2011 on the provision of food.
Nutrition Labelling - Centre for Food Safety Feb 1, 2021 ... Nutrition label must include the information on energy and seven nutrients specified for labelling (1+7), namely, protein, carbohydrates, ...
› research › healthBreastfeeding State Laws - National Conference of State ... Aug 26, 2021 · Ind. Code Ann. § 16-41-6-11 (2003) states that the state department should maintain rules that set forth standards to provide to women who are pregnant, before delivery, at delivery, and after delivery, information concerning HIV. The rules must include information about the risk of transmission through breastfeeding.
eCFR :: 21 CFR Part 312 -- Investigational New Drug Application A reference to information submitted previously must identify the file by name, reference number, volume, and page number where the information can be found. A reference to information submitted to the agency by a person other than the sponsor is required to contain a written statement that authorizes the reference and that is signed by the person who submitted …
Nutrition Final Exam Flashcards - Quizlet Information that must be lawfully provided on food labels includes all of the following except... The amount recommended for ingestion each day.
› current › title-21eCFR :: 21 CFR Part 812 -- Investigational Device Exemptions When the sponsor receives from the IRB information concerning the public disclosures under § 50.24(a)(7)(ii) and (a)(7)(iii) of this chapter, the sponsor shall promptly submit to the IDE file and to Docket Number 95S-0158 in the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD ...
› news-events › public-health-focusFDA Regulation of Cannabis and Cannabis-Derived Products ... In addition, under 21 CFR 530.20(b)(2), if scientific information on the human food safety aspect of the use of the approved human drug in food-producing animals is not available, the veterinarian ...





















Post a Comment for "42 information that must be lawfully provided on food labels"