38 phase labels in chemical equations
Design equations of Rectangular Microstrip Patch Antenna 17.5.2020 · Chemical Waste: A. Discarded disinfectants, Cleaning agents B. X-ray film developing liquid, Infected secretions C. Aspirated body fluids, Liquid from laboratories 6. Microbiology, Biotechnology, and other clinical lab waste 7. Chemical Liquid Waste Yellow bin/ bag with the cytotoxic label: 1. End-of-Chapter Material | Introductory Chemistry Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
Chemical Equations - GitHub Pages It is not uncommon to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note

Phase labels in chemical equations
End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl 2 (g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O (ℓ) should not be considered a proper chemical equation. Phase Rule - Teaching Phase Equilibria The system is entirely composed of H 2 O, so there is only one component present.; The phases present represent three states of matter: liquid (water), solid (ice), and vapor (steam). All have distinct physical properties (e.g. density, structure--or lack of, etc.) and chemical properties (e.g. Δ G formation, molar volume etc.) so they must be considered distinct phases. 3 Steps for Balancing Chemical Equations - ThoughtCo To do this, you need to be familiar with the properties of various compounds or you need to be told what the phases are for the chemicals in the reaction. Oxides are solids, hydrogen forms a diatomic gas, tin is a solid, and the term ' water vapor ' indicates that water is in the gas phase: SnO 2 (s) + 2 H 2 (g) → Sn (s) + 2 H 2 O (g)
Phase labels in chemical equations. 5.1 The Chemical Equation - Introductory Chemistry - 1st Canadian ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2 NaHCO 3 (s) →200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O(ℓ) State symbols and phase changes - StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l) The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways
Book Title: Introductory Chemistry – 1st Canadian Edition 16.9.2014 · Book Description: The goal of this textbook is not to make you an expert. True expertise in any field is a years-long endeavor. Here I will survey some of the basic topics of chemistry. This survey should give you enough knowledge to appreciate the impact of chemistry in everyday life and, if necessary, prepare you for additional instruction in chemistry. 5.2 Chemical Equations - Lumen Learning It is not uncommon to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note What are Chemical Equations? Detailed Explanation, Examples The reactants and the products (for which the chemical formulae are written in chemical equations) can be separated by one of the following four symbols. In order to describe a net forward reaction, the symbol '→' is used. In order to describe a state of chemical equilibrium, the symbol '⇌' is used. PDF Chapter 8 chemical equations and reactions test answer key Chemical equations can also be used to represent physical processes. Write a chemical reaction to boiling water, including the appropriate phase labels. Chemical equations can also be used to represent physical processes. Write a chemical reaction to freezing water, including the appropriate phase labels. Explain why 4NA (s) + Ã, 2cl2 (g) Ã, Ã,
PDF Strategy to Determine the Phase of a Chemical Mixture Ph or phase nc is the number of components Pure-Component Mixture (nc= 1, a pure compound ) For P= 1 atm, find T mand T bin Table B.1 of F&R, 3rdEd. If T > T b, then Ph= vapor where is T b the boiling point If T = T b, then Ph= vapor-liquid If T m< T < T 5.7 End-of-Chapter Material - Introductory Chemistry - 1st Canadian ... Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation. End-of-Chapter Material - GitHub Pages Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation. Solved Phase labels never appear in balanced chemical | Chegg.com Question: Phase labels never appear in balanced chemical equations true or false ? This problem has been solved! See the answer See the answer See the answer done loading
Conventions for Writing Chemical Equations - Arrows, Phases ... presents: Conventions for Writing Chemical Equations including Arrows, Phases, Coefficients, Reactants, Products and moreTired...
PDF aq - Anoka-Ramsey Community College • Predict possible products with phase labels • Write the balanced chemical equation. If no visible reaction occurs, write NR. If a precipitate forms, give the formula and name of the precipitate. • Write the complete ionic equation and net ionic equation for all reactions (whether or not a visible reaction occurs) 1.
chemical equation | Yeah Chemistry Formulas of the reactants appear on the left side of the equation; those of products are written on the right. In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution
Phase Diagram | Explanation, Definition, Summary & Facts A phase has a complete distinct physical and chemical properties, whereas two phases are separated by a phase boundary. A phase diagram is a graphical representation of the substance phases, consists of the curved lines and the space between the two lines represent a specific phase of the matter at given pressure and temperature, whereas any ...
Phase Definition and Examples - ThoughtCo In chemistry and physics, a phase is a physically distinctive form of matter, such as a solid, liquid, gas, or plasma. A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter.
Phase Diagrams - Chemistry - University of Hawaiʻi Using the phase diagram for water given in [link], determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa Solution
7.3: The Chemical Equation - Chemistry LibreTexts In the symbolic equation, chemical formulas are used instead of chemical names for reactants and products, while symbols are used to indicate the phase of each substance. It should be apparent that the chemical shorthand method is the quickest and clearest method for writing chemical equations.
Solved 1. Write a balanced thermochemical equation with | Chegg.com Question: 1. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation. (3 pts) 2. What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react? (9 pts) 3.
Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ...
EXAM #2: Chapter 4 Flashcards | Quizlet Write a chemical reaction for the boiling of water, including the proper phase labels. ... 2. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. ... 3. Explain why 4Na (s)+2ClX2 (g) 4NaCl (s) should not be considered a proper chemical equation.
End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
3 Steps for Balancing Chemical Equations - ThoughtCo To do this, you need to be familiar with the properties of various compounds or you need to be told what the phases are for the chemicals in the reaction. Oxides are solids, hydrogen forms a diatomic gas, tin is a solid, and the term ' water vapor ' indicates that water is in the gas phase: SnO 2 (s) + 2 H 2 (g) → Sn (s) + 2 H 2 O (g)
Phase Rule - Teaching Phase Equilibria The system is entirely composed of H 2 O, so there is only one component present.; The phases present represent three states of matter: liquid (water), solid (ice), and vapor (steam). All have distinct physical properties (e.g. density, structure--or lack of, etc.) and chemical properties (e.g. Δ G formation, molar volume etc.) so they must be considered distinct phases.
End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl 2 (g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O (ℓ) should not be considered a proper chemical equation.

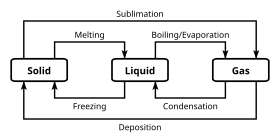


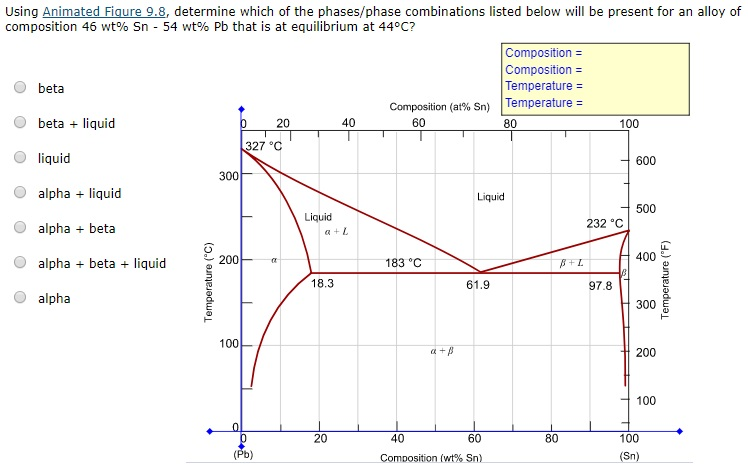








Post a Comment for "38 phase labels in chemical equations"